Cassava Sciences
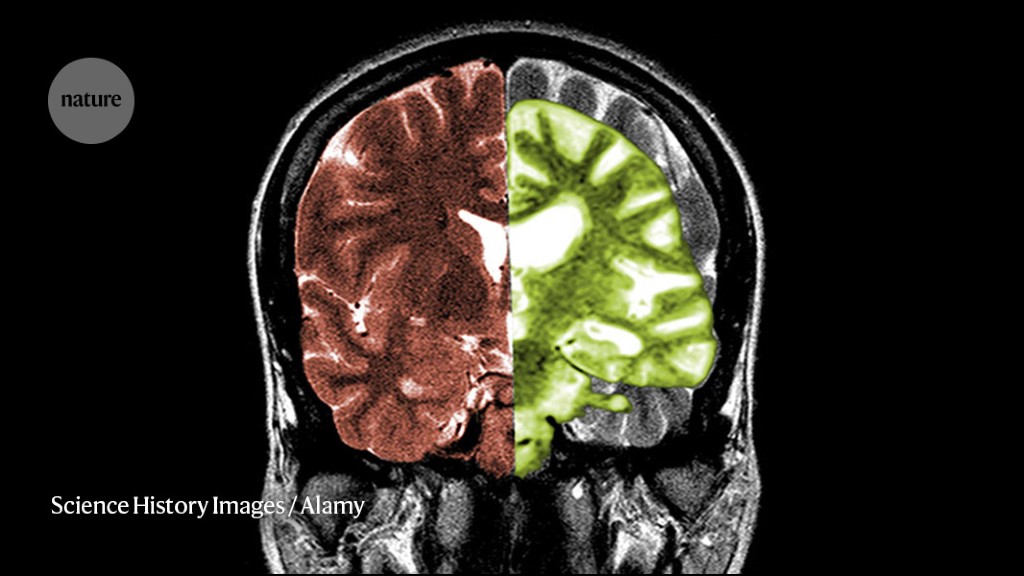
Cassava Sciences is an American pharmaceutical company based in Austin, Texas. The company was founded in 1998 by chief executive officer and president Remi Barbier as Pain Therapeutics, Inc., changing its name in 2019. Cassava is developing simufilam, an oral-tablet drug candidate for the treatment of Alzheimer's disease; simufilam is in phase III clinical trials as of 2022. Reuters reported on July 27, 2022, that the United States Department of Justice is investigating Cassava Sciences over research results related to the experimental drug. A citizen petition attempting to suspend the clinical trials was filed with the Food and Drug Administration, but the FDA said that the citizen petition "was not a proper avenue" to stop the trials in February 2022. Cassava Sciences has denied any wrongdoing and raised concerns about the motivations behind the FDA petition.